Animals and ethics
This study was conducted using adult inbred BALB/c mice obtained from a certified breeding colony. Eight-week-old male mice (mean ± SD body weight: 30 ± 2 g) and adult female mice (mean ± SD body weight: 26 ± 2 g) were used. All animals were housed under standard laboratory conditions, including a 12:12 h light-dark cycle controlled by an automatic lighting system and a thermoneutral temperature range of 21–23 °C maintained by automated air-conditioning units. Males and females were housed in separate rooms but under identical husbandry conditions. All animal procedures were approved by the Ethics Committee of the School of Veterinary Medicine, Shiraz University (ethics code: 4687/63) and complied with the European Council Directive 2010/63/EU on the protection of animals used for scientific purposes. The study was designed, conducted, and reported in accordance with the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments). At the end of the experimental period, mice were euthanized by gradual-fill CO₂ inhalation (20–30% chamber volume per minute) in a dedicated CO₂ euthanasia chamber, followed by cervical dislocation performed by trained personnel to ensure death, in accordance with the AVMA Guidelines for the Euthanasia of Animals (2020).
Experimental design
A total of 120 adult male BALB/c mice were randomly allocated into four experimental groups (n = 30 per group; 10 mice per cage): Control, Heat Control, Niacin 1, and Niacin 2. Group sizes were determined based on previous studies evaluating testicular damage and fertility outcomes under heat or reproductive stress conditions, ensuring adequate statistical power to detect meaningful differences22,67 and consistent with similar experimental designs in reproductive stress studies68. Sample sizes for each analysis were selected according to expected effect sizes and variability reported in inbred BALB/c mice: biochemical, hormonal, and oxidative stress assays (n = 7 per group) allow detection of ~ 25% differences with SD’s of 10–15% at ≥ 80% power and α = 0.05; molecular analyses (n = 6 per group) detect 1.5–2 fold changes with SDs of 0.3–0.4; histological and sperm assessments (n = 7 per group) detect ≥ 15% differences in motility or ≥ 1.5-unit changes in Johnsen scores; in vivo and in vitro fertility endpoints (n = 3 males per group) were based on previous reports showing that two females per male or ≥ 50 oocytes per male are sufficient to detect biologically meaningful differences under standardized conditions. The study included two sampling time points, on days 15 and 30 of systemic heat stress exposure. This design enabled temporal assessment of physiological, molecular, and fertility-related parameters under different treatment conditions.
All groups received a daily oral gavage of 200 µL sterile phosphate-buffered saline (PBS; calcium- and magnesium-free, Kalazist Company, Iran). In the Niacin 1 and Niacin 2 groups, PBS was supplemented with niacin (nicotinic acid; Sigma, NO761-100G) at doses of 100 mg/kg/day (Niacin 1) and 200 mg/kg/day (Niacin 2), corresponding to approximately 4 mg and 8 mg per mouse per day based on average body weights (20–40 g). These doses were selected using allometric scaling from human equivalent doses (500–1000 mg/day for a 65 kg adult) and guided by previous rodent studies demonstrating dose-dependent reproductive effects and safety69,70.
Heat stress was applied to the Heat Control, Niacin 1, and Niacin 2 groups by exposing the animals to 36 ± 2 °C and 55% relative humidity for 4 h daily (10:00–14:00) over 30 consecutive days71, following methodologies validated in rodent and rat studies assessing testicular ischemia-reperfusion and reproductive stress models25,50,72,73. Heat exposure began 30 min after gavage. The selected temperature was well above the thermoneutral zone to ensure exposure to heat stress, while the 4-hour duration was sub-lethal, sufficient to induce chronic stress without causing acute mortality. The Control group and all female mice were maintained under thermoneutral conditions throughout the study. Female mice were used exclusively under thermoneutral conditions for both in vitro and in vivo fertility evaluations.
Animal monitoring
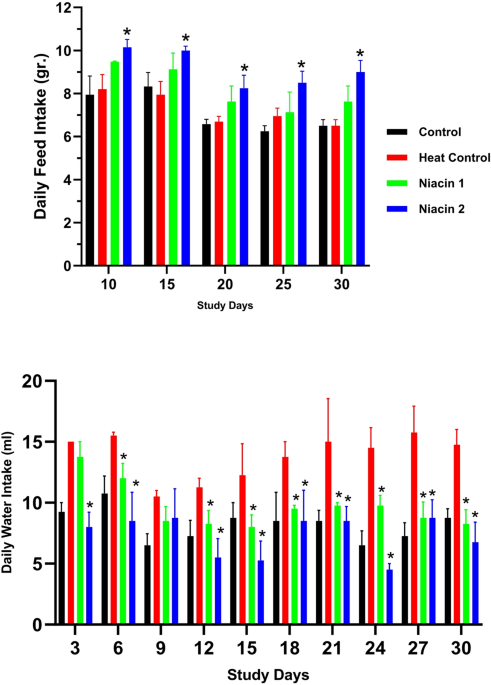
All mice had ad libitum access to commercial pelleted feed (21-Beiza Company, Fars, Iran) and fresh water provided via graduated bottles. Feed and water intake were recorded daily to monitor nutritional status and detect any changes due to treatment or heat exposure. To assess physiological responses to heat stress, body temperature was measured daily at 12:00, the midpoint of the heat exposure period. Rectal temperature was recorded using a pediatric digital thermometer with a flexible tip (Carent©), and abdominal surface temperature was measured using a non-contact infrared thermometer (NEUTRAL DT-8806©, CEM Instruments, Shenzhen, China) held 2 cm above the skin. All measurements were performed under minimal-stress conditions to avoid confounding effects.
Andrology assessments
Sample collection, timing, and organ dissection
Andrological parameters were evaluated at two time points: day 15 and day 30 of the experimental period. On each day, seven male mice from each group were randomly selected and euthanized by CO₂ inhalation followed by cervical dislocation at 08:00 AM, prior to daily heat exposure. Immediately postmortem, the testes were exposed, and the cauda epididymides were carefully excised and transferred to sperm analysis medium for evaluation. The abdominal cavity was then opened through a midline incision, and the reproductive tract was exposed. The seminal vesicles and prostate glands were identified and dissected under a stereomicroscope. The seminal vesicles were isolated intact, avoiding rupture of the glandular contents, and the entire prostate complex (including ventral, dorsal, and lateral lobes) was excised with minimal surrounding connective tissue. Additionally, the left testis was carefully removed after blunt dissection of the surrounding fat and epididymis. All tissues were briefly rinsed in ice-cold phosphate-buffered saline (PBS) to remove blood residues, then gently blotted on sterile filter paper to eliminate surface moisture. Wet combined weights of the seminal vesicles and prostate, as well as left testis were immediately measured using an analytical balance with 0.1 mg precision. The weights of each organ were recorded individually, and the combined weight of the seminal vesicles and prostate was also calculated74.
Total epididymal sperm count
For sperm collection, each cauda epididymis was finely minced into multiple pieces using sterile scissors and placed into sterile multi-well ELISA plates containing 100 µL of pre-warmed (37 °C) sperm analysis medium. The medium consisted of Tris (3.025 g), citrate (1.7 g), glucose (1.25 g), crystalline penicillin G (150,000 U), and crystalline streptomycin sulfate (150 mg), dissolved in distilled water to a final volume of 100 mL (pH = 6.76; osmolarity = 330 mosmol/kg H₂O), following methods previously validated for sperm viability and motility in small mammals75. Samples were incubated undisturbed on a 37 °C heating stage for 15 min before further analysis. Total sperm count was then determined using a hemocytometer under a light microscope.
Epididymal sperm motion kinematics
Sperm motility and motion characteristics were assessed using a computer-assisted semen analysis (CASA) system (HFT™ CASA System, HFT Co., Tehran, Iran) coupled with an Olympus bright-field microscope (Olympus, Tokyo, Japan) at 100× magnification. Approximately 5 µL of the sperm suspension was transferred into a pre-warmed (37 °C) counting chamber (Sperm 360 labs™, India) and immediately analyzed. At least five fields per sample were examined, with a minimum of 400 sperm cells analyzed across four random microscopic fields. Parameters evaluated included total motility (TM, %), progressive motility (PM, %), curvilinear velocity (VCL, µm/s), straight-line velocity (VSL, µm/s), average path velocity (VAP, µm/s), linearity (LIN, %), wobble (WOB, %), straightness (STR, %), mean angular displacement (MAD, °), amplitude of lateral head displacement (ALH, µm), and beat cross frequency (BCF, Hz). Sperm cells with a VAP < 10 μm/s were considered immotile. The CASA system operated at a frame rate of 50 Hz. Detailed technical settings used for the CASA analysis are provided in Table 10. All CASA parameters were adapted and validated specifically for BALB/c mouse sperm. Pilot experiments were conducted to optimize motility and velocity thresholds, ensuring accurate classification of motile, progressive, and immotile sperm. Calibration with reference sperm samples confirmed reproducibility, and all technical settings were applied consistently across experimental groups. This validation ensures that CASA measurements accurately reflect mouse-specific sperm motility and motion characteristics.
Sperm viability
Sperm viability was assessed using Eosin-Nigrosin (EN) staining. Ten microliters of sperm suspension were mixed with an equal volume of 1% eosin Y and 10% nigrosin in 2.9% sodium citrate solution, smeared on a clean glass slide, air-dried, and examined under a light microscope at 1000× magnification. Live sperm excluded eosin and appeared white, while dead sperm absorbed eosin and appeared pink or red. At least 200 spermatozoa were counted per slide.
Membrane functional integrity
The hypo-osmotic swelling test (HOST) was used to assess sperm plasma membrane functional integrity. Ten microliters of sperm suspension were incubated in 100 µL of hypo-osmotic solution (75 mOsm/kg; sodium citrate 7.35 g/L and fructose 13.51 g/L) at 37 °C for 30 min. Afterwards, a smear was prepared and evaluated under light microscopy. Sperm exhibiting swollen or coiled tails were considered HOST-positive. A minimum of 200 sperm were assessed per sample.
Acrosome integrity (coomassie brilliant blue staining)
To evaluate acrosome integrity, air-dried sperm smears were fixed in methanol for 10 min, then stained with 0.22% Coomassie Brilliant Blue G-250 in 50% methanol and 10% glacial acetic acid for 2 min. Slides were rinsed and examined at 1000× magnification. Intact acrosomes stained blue over the apical region, while damaged acrosomes showed partial or no staining.
Acrosome status (CTC staining)
Capacitation and acrosome reaction status were evaluated using chlortetracycline (CTC) staining. Spermatozoa were fixed in 4% paraformaldehyde and incubated in CTC solution (750 µM CTC, 130 mM NaCl, 5 mM cysteine, 20 mM Tris-HCl; pH 7.8) for 20 min in the dark. Slides were examined using a fluorescence microscope. Based on fluorescence patterns, sperm were classified as: F pattern: non-capacitated (uniform bright fluorescence over head), B pattern: capacitated (fluorescence-free band in post-acrosomal region) AR pattern: acrosome-reacted (dim or absent fluorescence over head).
DNA integrity (sperm chromatin dispersion assay)
DNA fragmentation was evaluated using the sperm chromatin dispersion assay (SCDA), modified for light microscopy with Diff-Quik staining. Briefly, spermatozoa were embedded in low-melting-point agarose on pre-coated glass slides and allowed to solidify. Slides were then incubated in an acid denaturation solution (0.08 N HCl) for 7 min at room temperature, followed by a lysis solution (containing 2.5 M NaCl, 100 mM EDTA, 10 mM Tris-HCl, and 1% SDS; pH 7.4) for 25 min. After washing and drying, the slides were stained with Diff-Quik (HFT Co., Tehran, Iran) according to the manufacturer’s protocol. Spermatozoa were examined under a light microscope at 1000× magnification. Nuclei with large and dispersed halos of chromatin around the core were classified as non-fragmented (intact DNA), whereas those with small or no halos were considered fragmented. A minimum of 200 sperm cells were evaluated per sample.
DNA maturation (acridine orange staining)
Sperm nuclear protamine deficiency was assessed using acridine orange (AO) staining. Air-dried smears were fixed in Carnoy’s solution (methanol: acetic acid, 3:1) for 2 h, stained with AO solution (0.19 mg/mL AO in phosphate-citrate buffer, pH 2.5) for 5 min, and immediately examined under a fluorescence microscope. Mature sperm chromatin fluoresced green (double-stranded DNA), while immature or protamine-deficient sperm showed yellow to red fluorescence (single-stranded or denatured DNA).
Endocrinology and hepatic function tests
Sample collection and preparation
Immediately upon sacrifice, blood samples were collected from all animals via direct collection from the heart chambers at Days 15 and 30 into sterile tubes without anticoagulants. After clotting for 30 min at room temperature, samples were centrifuged at 3000 rpm for 15 min to separate serum, which was aliquoted and stored at − 20 °C. Testis tissues were excised immediately after euthanasia, rinsed with cold phosphate-buffered saline (PBS), blotted dry, and stored at − 80 °C. For testosterone assays, tissues were thawed, homogenized in ice-cold PBS (1:10 w/v), centrifuged at 12,000 × g for 15 min at 4 °C, and the supernatants stored at − 20 °C.
Biochemical analysis of hepatic enzymes
Serum activities of AST, ALT, and ALP were measured using Bio-Rex commercial kits following the manufacturer’s instructions. AST and ALT activities were determined by monitoring NADH oxidation spectrophotometrically at 340 nm after enzymatic substrate conversion. ALP activity was assessed by measuring the hydrolysis of p-nitrophenyl phosphate to p-nitrophenol at 405 nm. Quality controls and calibrators were included in each run.
Measurement of thyroid hormones (T3 and T4)
Serum T3 and T4 concentrations were quantified using Denazist ELISA kits. Samples, standards, and controls (100 µL/well) were added in duplicate to antibody-coated microplates and incubated at 37 °C for 60 min. After washing, biotinylated detection antibodies and streptavidin-HRP conjugate were sequentially added with incubation steps. Color development was achieved using TMB substrate, stopped with acid, and absorbance measured at 450 nm. Hormone levels were calculated from standard curves.
Determination of serum and testis testosterone
Testosterone in serum and testis homogenates was measured using the Accubind Testosterone ELISA kit. Samples and standards (100 µL/well) were added to testosterone antibody-coated wells and incubated for 60 min at room temperature. Following washes, HRP-labeled enzyme conjugate and TMB substrate were added with appropriate incubations. The reaction was stopped with acid, and absorbance read at 450 nm. Concentrations were determined via standard curves. All assays were run in duplicates with intra- and inter-assay variability below 10%.
Oxidative stress biomarker assessments
Blood and testicular tissue samples were collected from male mice (n = 6 per group) on Days 15 and 30. Mice were euthanized using CO₂ gas followed by cervical dislocation at 08:00 prior to heat stress exposure. Blood was collected by cardiac puncture and allowed to clot for 30 min at room temperature. Serum was separated by centrifugation at 3000 × g for 10 min and stored at − 80 °C. Testes were excised, washed with ice-cold saline, weighed, and homogenized in cold phosphate-buffered saline (10% w/v). Homogenates were centrifuged at 10,000 × g for 15 min at 4 °C, and supernatants stored at − 80 °C. All oxidative stress markers were measured using commercial ZellBio GmbH assay kits (Ulm, Germany), strictly following the manufacturer’s protocols.
Malondialdehyde (MDA) assay
Serum and testicular MDA levels were quantified using the ZellBio TBARS (thiobarbituric acid reactive substances) assay kit (Catalog No. ZB-MDA96). For serum, 100 µL of each sample or standard was mixed with 100 µL of thiobarbituric acid (TBA) reagent in microcentrifuge tubes. For testicular tissue, excised testes were washed in ice-cold saline, weighed, and homogenized in cold phosphate-buffered saline (10% w/v). Homogenates were centrifuged at 10,000 × g for 15 min at 4 °C and the supernatant collected. Then, 100 µL of testicular supernatant was mixed with 100 µL TBA reagent. Samples were incubated at 95 °C for 40 min to allow formation of MDA-TBA adduct, cooled on ice, centrifuged at 4000 × g for 10 min, and absorbance measured at 535 nm using a microplate reader. MDA concentration was determined from a standard curve and expressed as nmol/mL for serum and nmol/g tissue for testis.
Total antioxidant capacity (TAC) assay
TAC was measured using the ZellBio TAC assay kit (Catalog No. ZB-TAC96), based on the Trolox equivalent antioxidant capacity method. Twenty microliters of serum were added into each well of a 96-well plate, followed by 200 µL of pre-mixed TAC reaction solution (containing chromogenic substrate and assay buffer). The plate was incubated at room temperature for 10 min protected from light. Absorbance was read at 593 nm. Trolox standards provided in the kit were used to generate a calibration curve. TAC values were expressed as mmol Trolox equivalents per liter of serum.
Superoxide dismutase (SOD) activity assay
SOD activity was assessed with the ZellBio SOD assay kit (Catalog No. ZB-SOD96), which uses a colorimetric method based on the inhibition of formazan dye formation by superoxide radicals. Twenty microliters of serum were added to 200 µL of WST-1 working solution and enzyme working solution in a 96-well plate according to the kit protocol. After incubation at 37 °C for 20 min, absorbance was measured at 450 nm. SOD activity was calculated based on the percentage inhibition of WST-1 reduction compared to a blank control, and expressed in units per milliliter (U/mL), where one unit corresponds to 50% inhibition of superoxide-induced formazan dye formation. All samples were analyzed in duplicate. Standard curves and blank controls were included in each assay run. Intra-assay coefficients of variation were maintained below 10%. Assays were performed with reagents and samples equilibrated to room temperature to ensure consistency.
Molecular assay
RNA isolation, cDNA synthesis, and quantitative real-time PCR
Immediately after sacrifice, testicles were snap-frozen in liquid nitrogen and stored at − 80 °C until use. For RNA extraction, tissues were thawed on ice in 500 µl of sterile, calcium- and magnesium-free phosphate-buffered saline (PBS; molecular grade, Shellmax, Cat. No. P1530-100 ml). Approximately 50 mg of tissue was transferred into sterile 1.5 ml RNase-free microcentrifuge tubes and homogenized on ice using a mechanical homogenizer with sterile plastic tips. One milliliter of ice-cold RNX™-PLUS reagent (CinnaGen, Iran) was added to each homogenized sample. Tubes were vortexed for 5–10 s and incubated at room temperature for 5 min. Then, 200 µl of chloroform was added, the tubes were gently shaken for 15 s (without vortexing), and incubated on ice or at 4 °C for 5 min. Samples were centrifuged at 12,000 × g for 15 min at 4 °C to achieve phase separation. The upper aqueous phase was carefully transferred to a new RNase-free tube, mixed with an equal volume of absolute ethanol, and applied in two steps to RNA spin columns (Pars Tous Co., Iran). The remaining steps were performed according to the manufacturer’s instructions. The purified RNA was eluted in 30 µl of nuclease-free water and stored at − 80 °C until use. RNA concentration and purity were assessed using a NanoDrop™ spectrophotometer (Thermo Fisher Scientific, USA) by measuring absorbance at 260 and 280 nm. cDNA synthesis was carried out using 600–700 ng of total RNA and the Easy cDNA Synthesis Kit (Pars Tous Co., Iran), utilizing both oligo(dT) and random hexamer primers, following the manufacturer’s protocol. Quantitative real-time PCR (qPCR) was performed to assess the expression levels of key genes involved in apoptosis (Bax, Bcl2, Casp3), heat stress response (Hspa1a/b), and cell communication (Gja1), with Actb used as the reference gene for normalization, following methods similar to those already described in oxidative-stressed testicular tissue and testicular stress models in rodents72,76. Gene-specific primers were designed using NCBI Primer-BLAST and synthesized commercially. Primer sequences, product sizes, and melting temperatures are provided in Table 11. qPCR was carried out using a SYBR Green detection system in 25 µl reactions containing 12.5 µl of RealQ Plus 2× Master Mix Green (Ampliqon™, Denmark), forward and reverse primers, 4 µl of diluted cDNA (15 ng/µl), and nuclease-free water. Amplification was performed using a Rotor-Gene Q real-time PCR cycler (Qiagen, Germany) under the following cycling conditions: initial denaturation at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 45 s. A melting curve analysis was conducted from 55 °C to 95 °C with 0.2 °C increments every 2 s to confirm the specificity of the amplified products. All samples were analyzed in triplicate. No-template and no-RT controls were included in each run to monitor for contamination and genomic DNA carryover. Relative expression levels were calculated using the 2^−ΔΔCt method. Primer efficiency was assessed using standard curves and confirmed to fall within the acceptable range of 90–110%.
In vitro fertility assay
Preparation of media
All culture media (T6, KSOM, and PB1; Table 12) were prepared in advance, sterile filtered, and equilibrated in a 5% CO₂ incubator at 37 °C for at least 4 h before use. All procedures involving gametes and embryos were performed under mineral oil (Sigma-Aldrich) in sterile 35-mm petri dishes (Nunc, Denmark) using a stereomicroscope in a laminar flow hood.
Sperm collection and capacitation
Upon sacrifice, male mice were placed in a sterile dissecting area. The cauda epididymides were immediately removed and placed in a sterile dish containing a 500 µl drop of pre-equilibrated T6 medium under mineral oil. Small incisions were made in the cauda regions using fine scissors and 26G needles to transfer the extracted sperm clot into the medium drop. The sperm suspension was incubated for at least 1.5 h at 37 °C in 5% CO₂ to allow for capacitation. Only the peripheral zone of the capacitation drop was used to collect motile sperm for IVF.
Superovulation and oocyte collection
Female mice were hormonally superovulated with 15 IU of human menopausal gonadotropin (HMG; Humegnan, Iran) administered intraperitoneally (i.p.) in a 100 µL volume at 5:00 PM, followed 50 h later by 10 IU of human chorionic gonadotropin (hCG; Gonarex, Iran) injected i.p. in 100 µL at 7:00 PM. Fifteen to sixteen hours after hCG administration, the females were euthanized using CO₂ inhalation. Both oviducts were carefully excised and placed in pre-warmed PB1 medium. The ampullary region was punctured using sterile fine-tipped needles to release cumulus-oocyte complexes (COCs), which were gently aspirated using finely drawn glass pipettes and transferred into pre-equilibrated 100 µL drops of T6 medium under mineral oil. COCs were washed 3–4 times and held in the incubator at 37 °C with 5% CO₂ until in vitro fertilization (IVF).
Fertilization procedure
Up to 50 COCs were placed in each 100 µL drop of T6 medium under mineral oil. Capacitated sperm were diluted in pre-equilibrated T6 to achieve a final concentration of 1 × 106 sperm/mL, following IVF protocols previously used for rodent sperm functional and fertility assessment77. A calculated volume of this sperm suspension was added to each fertilization drop containing COCs. The gametes were co-incubated at 37 °C in 5% CO₂ for 6 hours. After fertilization, cumulus cells were gently pipetted to aid removal if necessary. Presumptive zygotes were washed 3–5 times in clean pre-equilibrated KSOM drops to completely eliminate excess sperm and debris. Embryos were then transferred into fresh 100 µL KSOM drops under mineral oil for further development.
In vitro culture and developmental assessment
Embryos were cultured continuously for 5 days in the same conditions (37 °C, 5% CO₂, high humidity) without media change. Embryonic development was assessed at the following time points: 24 h post-insemination (Day 1): two-cell embryos (indicative of successful fertilization), 72 h post-insemination (Day 3): morula stage, 120 h post-insemination (Day 5): blastocyst stage. The number of embryos at each developmental stage was recorded, and representative images were captured using an inverted phase-contrast microscope equipped with a digital camera (e.g., Olympus IX71 with DP27 camera). Embryo development was expressed as a percentage of the initial number of oocytes cultured per group.
In vivo fertility assay
The in vivo fertility assay was conducted at the end of a 30-day treatment period using adult BALB/c mice. Female mice were synchronized using a modified hormonal protocol consisting of two intraperitoneal injections of PGF₂α (Dinoprost tromethamine, 10 µg/mouse) administered three days apart, with a single injection of progesterone (2 mg/mouse, s.c.) administered 24 h after the first PGF₂α dose, as described previously78. Vaginal cytology was performed daily using saline lavage and methylene blue staining to determine estrous stages. Only females identified in the estrus stage were selected for mating13. These females were housed with males from their respective treatment groups (2 females per 1 male) and cohabited for three consecutive days. The presence of a vaginal plug was checked each morning and considered evidence of mating. Plug-positive females were separated and monitored individually for pregnancy and delivery outcomes. Reproductive parameters recorded included pregnancy rate, litter size, stillbirth rate, and offspring sex ratio. Pup birthweights were measured within 24 h post-partum using a precision digital balance, and sex was determined based on external genital morphology.
Testicular histomorphometry
Sample collection and histological slide Preparation
At two designated time points (Day 15 and Day 30), five male mice from each experimental group were euthanized by CO₂ inhalation at 8:00 AM, prior to daily heat stress exposure. Immediately after euthanasia, the abdominal cavity was opened and both testes were carefully excised. Excess connective and adipose tissue were removed, and the testes were weighed using a precision analytical balance (± 0.001 g accuracy). The testes were then fixed in 10% neutral buffered formalin (pH 7.4) at room temperature for 72 h to preserve tissue architecture. The fixed testes were washed in PBS, dehydrated through graded ethanol, cleared in xylene, and embedded in paraffin using an automated processor. Paraffin blocks were trimmed and sectioned at 5 μm thickness, floated on a 40 °C water bath, mounted on slides, and dried overnight. Slides were then deparaffinized, rehydrated, stained with hematoxylin and eosin, dehydrated, cleared, and mounted with DPX (Di-styrene, Plasticizer, and Xylene).
Histomorphometry analyses
All stained slides were examined under a light microscope (Olympus BX51, Tokyo, Japan) connected to a digital camera (Dino-Lite, Taiwan) and a computer equipped with ImageJ software (version 1.53, NIH, USA) for morphometric measurements. For each testis, 25 randomly selected, round seminiferous tubule cross-sections with clear basement membranes and minimal distortion were chosen for analysis. The parameters measured included79:
• Seminiferous tubule diameter Two perpendicular diameters of each tubule were measured, and their mean was calculated to account for shape irregularities.
• Epithelial Thickness Measured as the linear distance from the basement membrane to the luminal border of the seminiferous epithelium at three equidistant points around each tubule and averaged.
• Spermatocyte Counts The number of spermatocytes per tubule cross-section was counted manually within the seminiferous epithelium.
• Interstitial Cell Counts Leydig and other interstitial cells were counted in at least twenty-five random, non-overlapping fields per testis at 400× magnification.
Histological Evaluation of Spermatogenic Function.
Three histological indices were used to assess spermatogenic function and quality79:
• Spermiogenesis Index (SI) Defined as the percentage of seminiferous tubules containing mature spermatids relative to the total number of tubules examined.
• Tubular Differentiation Index (TDI) Calculated as the percentage of seminiferous tubules containing more than three layers of differentiated germ cells, including spermatocytes and spermatids.
• Johnsen’s Score Each seminiferous tubule was assigned a score from 1 to 10 based on the degree of spermatogenic maturation and cellular organization, according to the Johnsen scoring system:
Score 10: Complete spermatogenesis with abundant spermatozoa.
Score 1: Absence of germ cells and complete tubular atrophy.
At least 100 seminiferous tubules were evaluated per animal, and mean values were calculated for each experimental group. Data were recorded using Microsoft Excel and subsequently analyzed statistically.
Statistical analyses
All statistical analyses were performed using SPSS software version 25 (IBM Corp., Armonk, NY, USA). Data were expressed as mean ± standard error of the mean (SEM). Normality of data distribution was assessed using the Shapiro-Wilk test. Depending on the experimental design, one-way or two-way repeated measures ANOVA was employed to evaluate differences among groups and across time points. When significant main effects or interactions were detected, post hoc comparisons were conducted using the least significant difference (LSD) test. Non-parametric variables, such as pregnancy rates and sex ratios, were analyzed using the Chi-square test or Fisher’s exact test as appropriate80. Statistical significance was set at P < 0.05 for all analyses.